
Two important properties involved in parts cleaning are viscosity and surface tension. Understanding these concepts is useful for determining what cleaning methods will be most effective for an application.
What is Viscosity?
Viscosity is a physical characteristic of fluids that demonstrates resistance to motion or flow. Water is an example of a low viscosity (thin) liquid, while toothpaste is an example of a high viscosity (thick) liquid.
Newtonian versus Non-Newtonian Fluids
Newton’s law of viscosity describes the relationship between the shear stress and shear rate of a liquid under a mechanical stress. Newtonian liquids obey Newton’s law of viscosity, which means that their internal flow resistance is independent of external force. In the case of non-Newtonian liquids, they can increase or decrease in viscosity as motion is increased.
Basically, viscosity is the resistance to motion of a liquid. For example, the viscosity of toothpaste is greater than that of motor oil, and the viscosity of motor oil is greater than that of water.
Toothpaste >
Motor Oil >
Water
In most cases viscosity decreases as temperature is increased, however motor oil is an example of an engineered fluid, which is formulated to maintain its viscosity.
Surface Tension
Surface tension describes the strength of a liquid at its surface. Surface tension is created as molecules at the surface of the liquid have a stronger attraction to each other than they do for the molecules that comprise the material next to it.
A higher the surface tension means a more stable barrier between two substances, which will prevent them from mixing. The surface tension of a liquid almost always decreases as the temperature of the liquid is increased. Chemical additives such as soaps may also reduce surface tension.
Surface tension provides a good means for gauging the effectiveness of many cleaning agents. One way of measuring surface tension is by identifying the contact angle where a drop of a liquid meets a surface. If the surface activity is known, the contact angle measures surface tension. If the surface tension of the liquid is known, the contact angle measures the activity of the surface.
Generally, we think of high viscosity liquids as having high surface tension and intuitively associate the two. However, a high viscosity liquid like wood glue actually has very low surface tension, enabling it to penetrate irregular wood surfaces.
The table below shows the viscosity and surface tension of some familiar liquids.
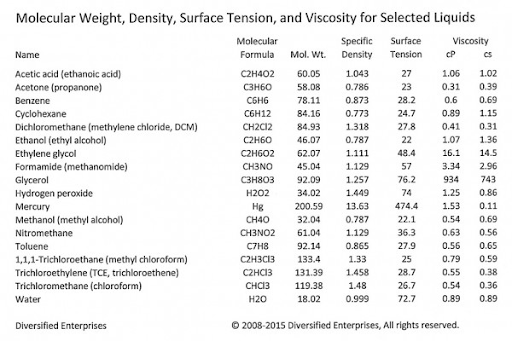
When considering viscosity and surface tension in parts cleaning, it is important to keep in mind that the two properties are different. Liquids with high surface tension and/or high viscosity can be difficult to remove from a surface. Liquids with low surface tension and low viscosity are good cleaners. Increasing temperature tends to make both properties more successful at cleaning.
Need assistance with selecting the right parts cleaning formulas and equipment? Contact us to discuss your application!



 English
English Spanish
Spanish Chinese
Chinese Canada
Canada Mexico
Mexico United Kingdom
United Kingdom




